Einzel- und Kombi-Impfungen im Vergleich
Ungenügende Vergleichsstudie zwischen 5fach-Impfstoff PEDIARIX und Einzelimpfungen
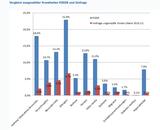
(ir) Barbara Fisher von der US-amerikanischen Impfkritiker-Organisation "National Vaccine Information Center" (NVIC) bezeichnete in ihrem Newsletter vom 8. Okt. 2007 eine Studie des größten europäischen Impfstoffherstellers GlaxoSmithKline (GSK) als mangelhaft. In dieser Studie wurden 575 Säuglinge im Alter von zwei, vier oder sechs Monaten entweder mit PEDIARIX, einem Fünffach-Impfstoff oder einzeln geimpft. Die Ergebnisse seien, so Fisher, aufgrund des schlechten Studiendesigns unbrauchbar, und allein schon deswegen sei die Impfung von gesunden Säuglingen irrwitzig:
1. Die Studiengruppe sei zu klein, um eine Aussage über die Häufigkeit von Impfkomplikationen treffen zu können.
2. Eine Nachverfolgung von sieben Monaten sei zu kurz. Fisher fordert einen Beobachtungszeitraum von fünf Jahren zur Beobachtung von Langzeitwirkungen.
3. Es fehle eine völlig ungeimpfte Kontrollgruppe, um vor allem neurologische Störungen, die von den Impfungen verursacht werden, realistisch einschätzen zu können.
NVIC E-news
Combo Vaccine Study Proves Nothing
by Barbara Loe Fisher
A study funded by British vaccine maker, GlaxoSmithKline, and conducted by University of Rochester vaccine developers claims to have proven that simultaneously injecting infants with 7 vaccines in separate shots is no more reactive or less effective than simultaneously injecting infants with 7 vaccines contained in combination shots. http://www.medicalnewstoday.com/articles/8460 6.php
Specifically, GSK conducted the study in an effort to "prove" that the 5 vaccines in 1 shot, Pediarix, can be given simultaneously with other vaccines without causing more reactions or compromising the effectiveness of the pertussis (whooping cough) portion of the shot as pre-licensure studies indicated. http://www.fda.gov/ohrms/dockets/ac/01/transcrip ts/3733t1.htm
What do GSK officials and University of Rochester doctors running the study for GSK think that they have proven about Pediarix safety and effectiveness? Do they really believe the educated public will be reassured by a study that only included 575 two month old healthy babies divided into three groups - all of whom got seven vaccines whether given separately or in combination?
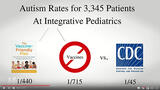
To accurately answer outstanding questions about Pediarix safety and effectiveness - as well as the safety and effectiveness of repeatedly injecting babies with seven vaccines simultaneously at two, four, and six months - GSK would have had to prospectively enroll at least 3,000 - 5,000 infants aged two months to five years and followed them up for at least five years. The study should also have included an unvaccinated group of children with a five year follow-up period to compare the brain and immune system function of unvaccinated children to those who were injected with Pediatrix in combination with other vaccines. By age five, the symptoms of ADHD, learning disabilities, autism, asthma, severe allergies and other neuroimmune dysfunction become apparent and, if autism occurs in about 10 per 1,000 children, a study of 3,000 to 5,000 children would yield between 30 and 50 autistic children by age five in all groups if there are no health outcome differences between vaccinated and unvaccinated children.
Even without an unvaccinated control group, it is ludicrous to conclude that studying 575 healthy newborns and following them up for 7 months generates enough useful data to conclude much of anything about giving infants so many vaccines on one day, whether the vaccines are given separately or in combination. This study by GSK to promote purchase and use of Pediarix does nothing to reassure parents and doctors that there are no adverse long term health consequences from repeatedly using this vaccine in combination with other pediatric vaccines in the first year of life.
Quelle: NVIC-Newsletter vom 8. Okt. 2007