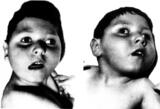
Krämpfe und Hautblutungen nach Impfung gegen Meningokokken C
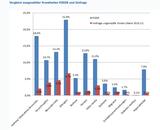
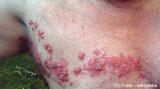
(ht) Eine britische Studie bestätigt Berichte, wonach die Impfung gegen Meningokokken-C erhöhte Risiken für Krämpfe und Hautblutungen in sich birgt. Allerdings versäumt es auch diese Studie, völlig Ungeimpfte mit in die Auswertung einzubeziehen. Statt dessen werden drei Impfungen miteinander verglichen.
Post-licensure safety of the meningococcal group C conjugate vaccine.
Passive surveillance reports of adverse events following meningococcal group C conjugate vaccine (MCCV) in the United Kingdom suggested a possible increased risk of convulsions and purpura. To investigate this further, hospital admissions for convulsions and purpura were obtained for the period November 1999 to September 2003 in children from the South East of England and these were linked to vaccine records for MCCV, Diphtheria/Tetanus/Pertussis vaccine (DTP) and Measles/Mumps/Rubella vaccine (MMR). A total of 1,715 children with convulsions and 363 with purpura were successfully linked to vaccination records. The self-controlled case-series method was then used to investigate whether there was any epidemiological evidence of an increased risk of convulsions or purpura following vaccination. The results showed that there was no evidence of an increased relative incidence (RI) of convulsions in the two weeks following MCCV with RI estimates (95% confidence intervals) of 0.57 (0.36-0.86), 1.03 (0.62-1.69) and 0.81 (0.51-1.30) for children aged <1, 1, 2-17 years respectively. There was also no increased relative incidence of purpura in the 4 weeks following MCCV, with an overall RI of 1.15 (0.80-1.67). There was evidence of an increased risk of convulsions and idiopathic thrombocytopenic purpura following MMR vaccination as previously documented. PMID: 17312400 - Hum Vaccin. 2007 Mar-Apr;3(2):59-63. Epub 2007 Mar 17