"USA: Nebenwirkungen von ROTATEQ werfen ernste Fragen auf"
 | |
| Das Impfrisiko ernst zu nehmen wird immer wichtiger. |
(ir) Einer neuen Veröffentlichung im "Medical Science Monitor" zufolge wirft eine Analyse der im Zusammenhang mit dem Rotavirus-Impfstoff ROTATEQ gemeldeten Nebenwirkungen ernste Fragen zur Verwendung des Impfstoffs in den USA auf.
RotaTeq vaccine adverse events and policy considerations.
Med Sci Monit. 2008 Mar;14(3):PH9-16
Authors: Geier DA, King PG, Sykes LK, Geier MR
http://lib.bioinfo.pl/pmid:18301365
Background: Rotavirus is the leading cause of severe gastroenteritis in children <5 years-old worldwide. On February 3, 2006, the US Food and Drug Administration licensed RotaTeqtrade mark (Merck and Co.), a bioengineered combination of five human-bovine hybridized reassortment rotaviruses. In August of 2006, the Advisory Committee on Immunization Practices recommended RotaTeq for routine vaccination of US infants administered orally at the ages 2, 4, and 6 months.<br />
Material/Methods: An evaluation of data reported to VAERS following the first five quarters of post-marketing surveillance of RotaTeq was undertaken. Trends in adverse events reported following RotaTeq and cost-effectiveness calculations of RotaTeq in the context of the disease burden of rotavirus in the US were examined.<br />
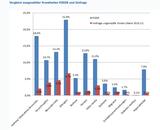
Results: From February 3, 2006 through July 31, 2007, a total of 160 (of the 165 reported) intussusception and 11 (of the 16 reported) Kawasaki disease adverse event reports were identified when RotaTeq was administered or co-administered with other vaccines. Time-trend analyses showed that there were significant increases in the total number of intussusception and Kawasaki disease adverse events entered into VAERS in comparison to previous years.<br />
Conclusions: These observations, coupled with limited rotavirus disease burden, cost-effectiveness, and potential contact viral transmission concerns, raise serious questions regarding the use of RotaTeq in the US. Healthcare providers should diligently report adverse events following RotaTeq vaccination to VAERS, and those who have experienced a vaccine-associated adverse event should be made aware that they may be eligible for compensation from the no-fault National Vaccine Injury Compensation Program (NVICP)